


-
-
Scientific LibraryAcute Coronary Syndrom ASCVD Prevention Bifurcation Stenting Cardio-Oncology Congestive Heart Failure DAPT Duration Drug Coated Balloon Fractional Flow ReserveNewsCases VideosE-LearningIndustry Insights
- LIVE
-
 Article Link
Article Link

How Will the Transition to hs-cTn Affect the Diagnosis of Type 1 and 2 MI?
Y Sandoval; FS Apple; AS Jaffe
KEYWORDS
Accidental Falls; Acute Coronary Syndrome; Angina; Unstable; Atrial Fibrillation; Angiography; Biological Markers; Cardiomyopathies; Chemistry; Clinical; Chest Pain; Confidence Intervals; Coronary Angiography; Coronary Artery Disease; Diabetes Mellitus; Type 2; Electrocardiography; Emergency Service; Hospital; Exercise Test; Heart Failure; Incidence; Interleukin-13; Kaplan-Meier Estimate; Kidney Diseases; Kidney Diseases; Myocardial Infarction; Myocardial Ischemia; Myocarditis; Myocardium; Patient Selection; Percutaneous Coronary Intervention; Prevalence; Point-of-Care Systems; Prospective Studies; Reference Values; Registries; Renal Insufficiency; Research Personnel; Thrombosis; Troponin I; Troponin T; United States Food and Drug Administration
Cardiac troponin (cTn) is the preferred cardiac biomarker used for the detection of myocardial injury.1 It can be used to facilitate the exclusion of acute myocardial infarction (MI) or its inclusion when there is a rising and/or falling pattern of cTn with at least one value >99th percentile.1 High-sensitivity cTn assays have been used clinically outside the United States for years. In January 2017, the US Food and Drug Administration (FDA) cleared the Elecsys Troponin T Gen 5 STAT assay (Roche Diagnostics; Indianapolis, IN) for clinical use. This assay was not designated as high-sensitivity by the FDA, and it is not clear that it meets the analytical high-sensitivity criteria suggested by the International Federation of Clinical Chemistry Task Force on Clinical Applications of Cardiac Bio-Markers (IFCC TF-CB).2-4 Despite that, it has been considered clinically to be a high-sensitivity assay in Europe and most of the rest of the world.5-6 There are four other assays that could be deemed "high-sensitivity" currently under review by the FDA for 510(k) clearance. In this document, we will use the term hs-cTn (both I and T) for the ease of our readers because the assays are being used clinically with protocols developed for high-sensitivity assays.
To understand the impact on the diagnosis of acute MI upon transition to hs-cTn assays, clinicians need to embrace some basic understanding of the assays. Detailed reviews of the analytical characteristics of hs-cTn assays have been published by the IFCC TF-CB.2,7-8 Basically, hs-cTn assays should allow
- the detection of very low cTn concentrations, i.e., superior analytical sensitivity, defined as the ability to measure cTn concentrations in ≥50% of "normal" individuals above the assay's limit of detection (requiring both men and women individually to attain such measurable concentrations [≥50% for men and ≥50% for women]), and
- improved imprecision (% coefficient of variation, ≤10%) at the 99th percentile for both sex-specific upper reference limits (URL).2,7-8
Figure 1
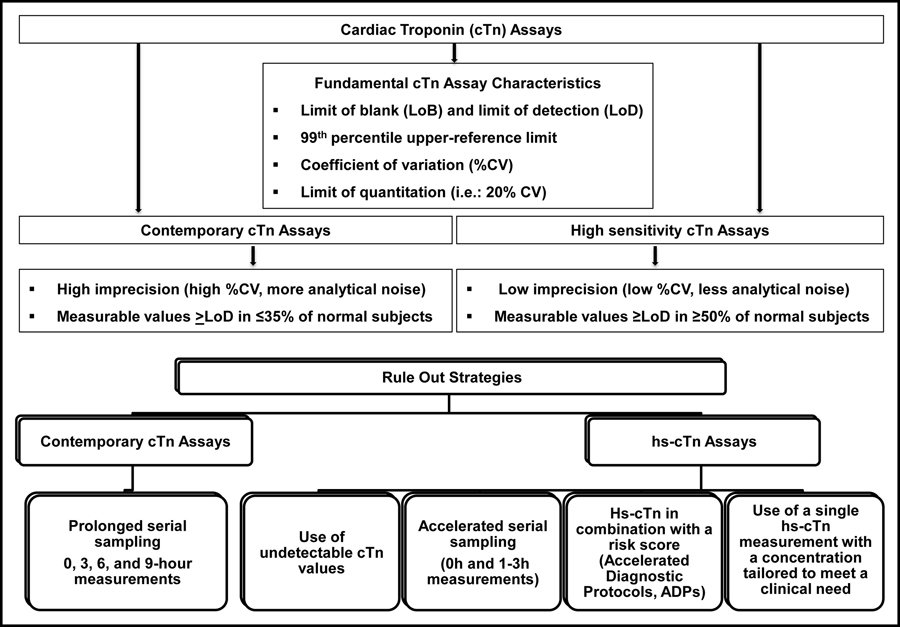
Fundamental cTn assay characteristics and diagnostic strategies comparing contemporary to hs-cTn assays (Reprinted with permission from Sandoval et al.) 9
How Will Transitioning to hs-cTn Affect the Diagnosis of Acute MI?
One of the common questions and potential concerns relates to whether there will be an increase in the proportion of patients with cTn concentrations >99th percentile and the diagnoses of acute MI when implementing a high-sensitivity assay. Data suggest that the impact largely depends upon the characteristics of the new assay and the sensitivity and diagnostic thresholds in use with the assay deployed prior to hs-cTn assay implementation.
In an analysis from the APACE (Advantageous Predictors of Acute Coronary Syndromes Evaluation) investigators, Reichlin et al. examined 1,124 consecutive patients presenting to the emergency department with symptoms suggestive of MI and demonstrated that 22% of the fourth generation Elecsys Troponin T STAT (Roche Diagnostics; Indianapolis, IN) concentrations were increased >99th percentile at presentation compared to 36% using the hs-cTnT assay.10 The fourth generation cTnT assay defined an incidence of acute MI and unstable angina of 18% and 13%, respectively, whereas the newer assay resulted in an increase of acute MI diagnoses to 22% and a reciprocal reduction in the rate of unstable angina to 11% (Figure 2).10 In another study by the same group,11 the rate of coronary angiography (23 vs. 23%, p = .092) and percentage of percutaneous coronary interventions (13% in both periods) were similar before and after introduction of hs-cTnT, with a substantial reduction in the use of stress tests (29 vs. 19%, p < 0.0001). This suggests that most of the patients newly included with the high-sensitivity assay were those previously categorized as having unstable angina with the fourth generation cTnT assay.
Figure 2
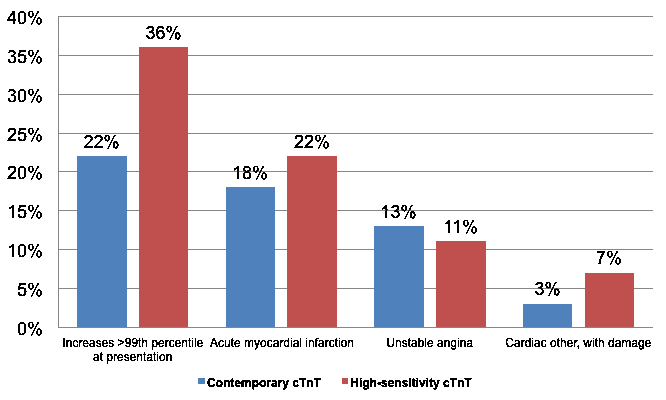
Incidence of increases >99th percentile at presentation, acute MI, unstable angina, and other cardiac conditions with evidence of damage using contemporary cTnT and hs-cTnT assays. (Figure adapted from Reichlin et al.) 10
In a large nationwide observational cohort study using the Swedish National Patient Registry, Odqvist et al. compared the incidence of MI early (within the 3 months before and after hs-cTnT implementation) after introduction of the hs-cTnT assay and demonstrated a 5% increase in the number of MI but no significant differences in the number of coronary angiographies (1.03; 95% confidence interval [CI], 0.97-1.09) or revascularizations (1.04; 95% CI, 0.98-1.11).12 Further analyses examining the subsequent years 2009-2013 suggested that small additional numbers of patients diagnosed using hs-cTnT were more likely to undergo angiography (adjusted hazard ratio [HR] 1.16; 95% CI, 1.14-1.18) and revascularization (adjusted HR 1.13; 95% CI, 1.11-1.15) within 30 days than those originally evaluated with the fourth generation cTnT assay, with no differences in all-cause mortality.12
Other studies using hs-cTnI assays have shown that the rate of cases with concentrations >99th percentile and/or diagnoses of acute MI may not increase with the use of high-sensitivity assays. The UTROPIA (Use of Abbott High Sensitivity Troponin I Assay In Acute Coronary Syndromes) study was a prospective cohort study of 1,640 patients presenting to an urban US emergency department who underwent serial cTnI measurements for clinical indications with both a contemporary cTnI (clinically used) and hs-cTnI assay (investigational, not FDA-approved but used globally) (both Abbott Laboratories; Chicago, IL).13 The number of patients with concentrations >99th percentile, as well as the incidence of acute MI, was significantly lower when using the hs-cTnI assay (driven by fewer type 2 MI) (Figure 3).13 Similar findings were shown by Greenslade et al. when comparing baseline samples at presentation between a contemporary cTnI assay for which 7.8% of patients had a value >99th percentile and a hs-cTnI assay for which 8.1% had a value >99th percentile (both Beckman Coulter Inc.; Brea, CA) (high-sensitivity assay not FDA-approved).14 It may well be that that these differences inform more about the sensitivity of the prior iteration of assays than the new assay being implemented.
Figure 3
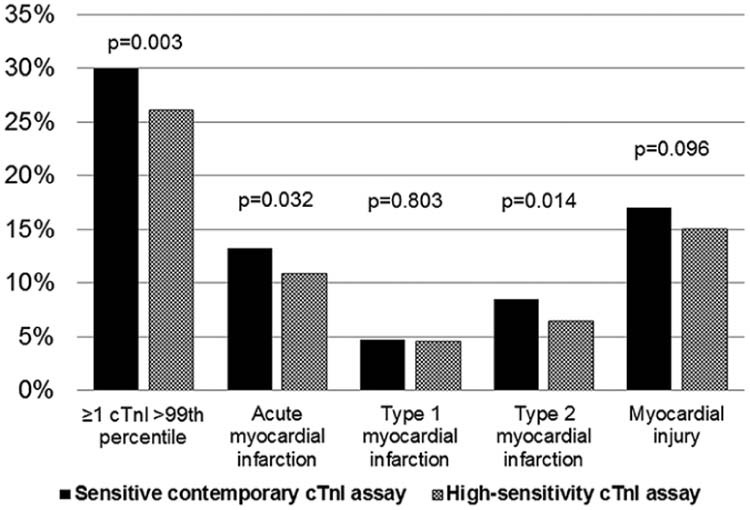
Incidence of myocardial necrosis (≥1 cTnI >99th percentile), acute MI, type 1 MI, type 2 MI, and myocardial injury using contemporary versus hs-cTnI assays (both Abbott Laboratories; Chicago, IL). (Reprinted with permission from Sandoval et al.) 13
Although it is tempting to think that transitioning to a high-sensitivity assay might lead to more MI diagnoses and increased values >99th percentile, matters are not that simple because, in part, the various assays available (I and T, point-of-care, contemporary, and high-sensitivity) differ in their analytical characteristics and URLs. How does one explain the fact that some studies do not show an increase in values >99th percentile (and consequently do not lead to more MI diagnoses) when using a high-sensitivity assay? One important fact is that MI itself usually causes more marked cTn increases than do many other causes of myocardial injury, so the impact of hs-cTn may be less. Data comparing cTnI to hs-cTnI (Abbott Laboratories; Chicago, IL) suggest an improved ability to quantify very low cTn concentrations within the normal range using the high-sensitivity assay compared with the contemporary assay without an increase in values >99th percentiles (Figure 4).15 In addition, the improved analytical imprecision obtained with high-sensitivity assays, particularly at the 99th percentile URL decision threshold may lead to an improved classification of cases, with those classified as "increased" with the contemporary assay likely being due to "analytical noise" when compared to the high-sensitivity assay (Figure 5).16 This has also been shown for hs-cTnT.17
Figure 4
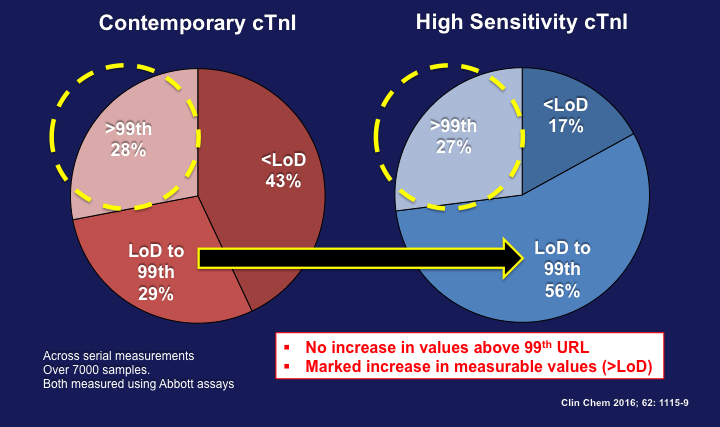
Comparison of cTnI concentrations across serial cTnI measurements using both contemporary and hs-cTnI assays (Abbott Laboratories; Chicago, IL). (Figure adapted from Love et al.) 15
Figure 5
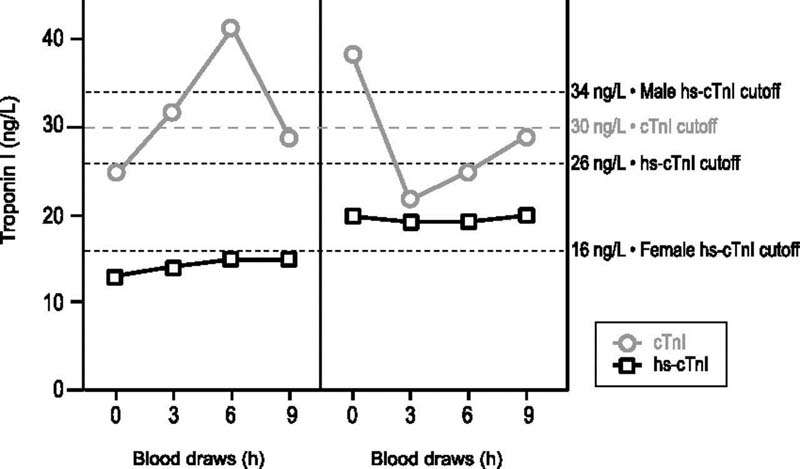
Impact of analytical variation on the diagnosis of acute myocardial injury as illustrated by serial cTnI concentrations in two patients measured by contemporary and hs-cTnI assays (Reprinted with permission from Sandoval et al.) 16
Data suggest that there should not be a universal increase in myocardial injury and MI diagnoses if a reasonably sensitive cTn assay and the appropriate 99th percentile had been used before high-sensitivity implementation. If so, little change may occur. However, for hospitals using insensitive assays or higher-than-recommended cutoff values, implementation may be coupled with a rise in the proportion of cases with increased concentrations >99th percentile and acute MI. Regardless of the situation, all sites will benefit from the more expeditious evaluations using high-sensitivity assays.
For hospitals in which the implementation of high-sensitivity assays translates to more cTn increases >99th percentile, it is important to indicate that these events are true positives for myocardial injury and will lead to the detection of new high-risk patients. Some will be diagnosed with acute MI previously not identified with contemporary cTn measurements (Figure 6), but most will represent changes in those diagnosed with unstable angina transforming to acute MI.10
Figure 6
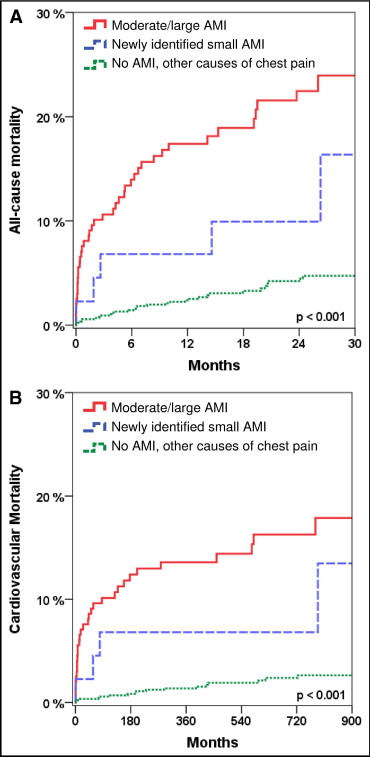
Kaplan-Meier analysis of outcome within 30 months. Analysis of all patients (n = 1124) stratified by moderate/large acute MI (as identified by fourth generation and hs-cTnT), newly identified small acute MI (as identified only by hs-cTnT), and no acute MI but other causes of chest pain regarding the endpoint of (A) all-cause mortality and (B) cardiovascular mortality. (Reprinted with permission from Reichlin et al.) 10
An important challenge for centers experiencing a rise in increases >99th percentile will be that most of these additional increases are not due to acute MI but rather to other pathologic etiologies. This is supported by numerous studies, including the analysis by the APACE investigators showing that the most pronounced increase in diagnoses was observed in patients with other cardiac conditions, such as myocarditis or acute heart failure (Figure 2).10 The SWEDEHEART (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) study involving 48,594 patients reported similar trends.18 Overall, 1 in 5 (21.6%) patients had an increase in hs-cTnT concentrations ranging from 14 to 49 ng/L, a concentration range where most patients could have had a negative cTnT using the older contemporary (fourth generation) cTnT assay. Of those, 18.2% were diagnosed with acute MI, but 38% had other conditions (heart failure, other cardiac diseases such as myocarditis or atrial fibrillation, and unknown or other non-cardiac disease) and a substantial percentage of other non-cardiac primary diseases.18 Importantly, there was a gradient of risk for mortality in each group predicated on the magnitude of the hs-cTnT increase.
For centers that will not experience an increase in cTn increases >99th percentile, the process may be less challenging upon adoption. However, it is important to emphasize that in US practice, most cTn increases will still be due to non-atherothrombotic conditions. Only about 15-18% of cases of cTn increases will be due to type 1 MI using either contemporary or hs-cTnI assays.13
Regardless of the assay (contemporary or high-sensitivity), the key in distinguishing these events is largely made on a clinical basis (Figure 7). The diagnosis of acute MI requires evidence of myocardial ischemia in addition to a rise and/or fall in cTn concentrations with at least one value >99th percentile (i.e., ischemic symptoms, new or presumed-new significant ST-T wave changes, development of pathological Q waves, imaging evidence of new loss of viable myocardium or new regional wall motion abnormalities, and/or identification of an intracoronary thrombus by angiography).1,19 Patients with type 1 MI are more likely to manifest larger concentration changes (as well as maximum concentrations) across serial measurements compared with patients with type 2 MI.20,21 However, serial concentration changes overlap, obviating the use of serial cTn changes (delta) as an aid in distinguishing type 1 from 2 MI.22
Figure 7
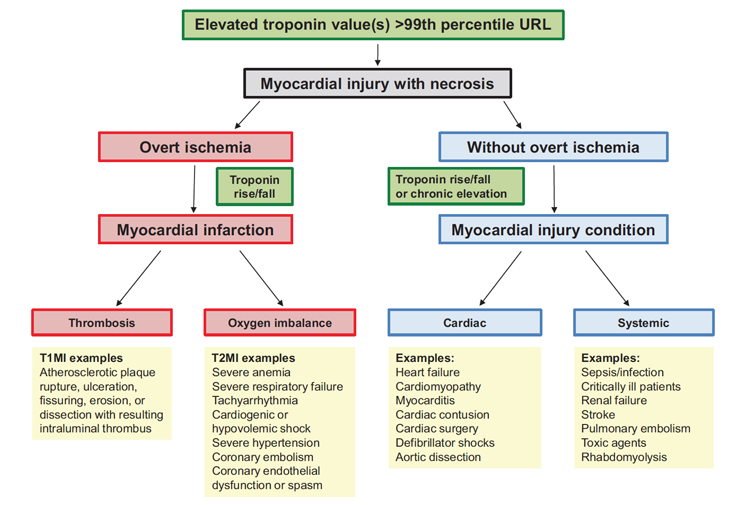
Conceptual model for myocardial injury and MI. (Reprinted with permission from Sandoval et al.) 19
Another issue that may affect the diagnosis of acute MI upon high-sensitivity assay implementation is the concentration URL used to detect myocardial injury. Despite guideline recommendations to use the 99th percentile as the preferred concentration threshold to diagnose acute MI, <50% of laboratories in North America use this decision threshold.23-26 The CARMAGE (Cardiac Marker Guideline Uptake in Europe) study surveyed European laboratories as well as practice patterns in North America and showed that only 45% of laboratories use the 99th percentile, with the remaining 55% using other non-guideline thresholds.25 A substantial group (32%) uses thresholds based on imprecision (i.e., 10 or 20% coefficient of variation), which means the use of concentration thresholds much higher than the 99th percentile.25Similar findings substantiate that only a minority of hospitals use the recommended 99th percentile.24,26 If a group is using cutoff values and/or insensitive assays and then implements hs-cTn assays, it is likely to find a marked increase in the frequency of increased values with the move to hs-cTn. That may not be due to the new assay as much as to the use of inappropriately high cutoff values with the prior assay.
An added challenge that may occur is when hospitals not currently using the 99th percentile transition to high-sensitivity assays and implement the use of sex-specific 99th percentiles as recommended by the IFCC TF-CB.2,7-8 The "positivity" rate may increase not exclusively because of using more sensitive assays, but also because of using lower decision thresholds that are appropriate for women.27 It has been shown that the implementation of a sensitive cTnI assay that involved lowering the diagnostic threshold for MI increases the rate of acute MI diagnoses and identifies patients at high risk, leading to the implementation of guideline recommended treatments with an associated significantly lower risk of death and recurrent MI.28 Analyses classifying events following the universal definition of MI shows that lowering the diagnostic threshold increases the diagnosis of type 2 MI or myocardial injury more than type 1 MI. Many of these patients are women. It also leads to an increase in healthcare resources that reduce MI and death for patients with type 1 MI but not for type 2 MI or myocardial injury.29
Finally, it is important to acknowledge how cTn testing is used in the US differs compared to the rest of the world (Figure 8).30 In a collaborative study evaluating how the selection of patients for hs-cTn testing affects the diagnosis of MI across different health care settings (United States and United Kingdom), Shah et al. showed that patients selected for cTn testing in the United States were much less likely to present with chest pain compared with those in the United Kingdom (51.2 vs. 83.0%). Perhaps for that reason, most cases with elevated hs-cTn in the United States are due to type 2 MI or myocardial injury, and hs-cTnI >99th percentile has a positive predictive value for type 1 MI of only 16.4%.30These data suggest that although hs-cTn assays will expedite the identification of patients at low risk who rule out for acute MI, to improve the rule-in efficiency, patient selection process may need to be refined to better integrate other features that improve the positive predictive value, such as the presence of chest pain, an ischemic electrocardiogram (ECG) or history of ischemic heart disease,30 or a higher absolute value for the European Society of Cardiology algorithm.5
Figure 8
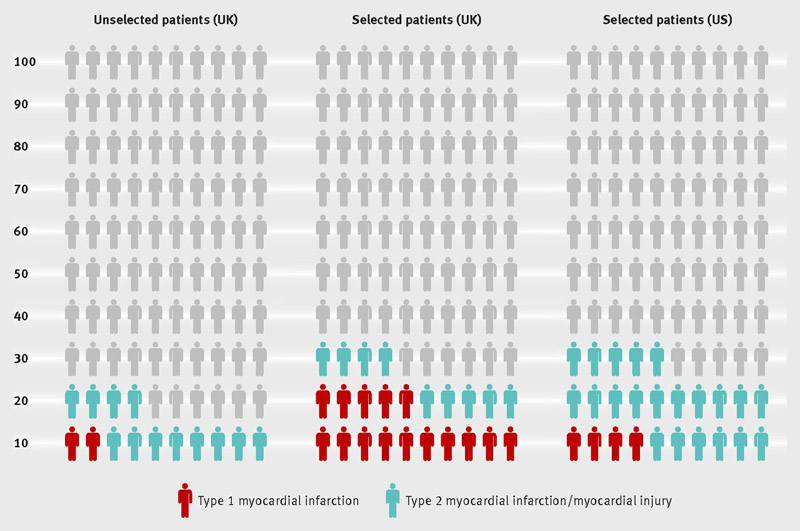
Prevalence of elevated hs-cTn concentrations and type 1 and 2 MI in unselected patients and those selected for cTn testing in the United Kingdom and United States. (Reprinted with permission from Shah et al.) 30
Of particular importance is the problem of late presenters. In one study, up to 26% of patients with acute MI did not have a rising and/or falling pattern detected likely because their values were declining on the downslope of the time-concentration curve, which is far slower than the upslope.31 Therefore, clinicians need to be sensitive to those who appear to have a substantial likelihood of acute MI and have elevated values that do not change after a short period of time. In those patients, a later value is likely to be helpful.5,31
In conclusion, studies have shown that the use of hs-cTn assays will expedite the evaluation of patients with suspected MI, with most cases able to be safely ruled-in or ruled-out within 1-3 hours, although some caution is advised in those who present early after symptom onset (<2-3 hours).9 The implementation of high-sensitivity assays in the United States will require multidisciplinary efforts and collaboration among cardiologists, emergency physicians, and laboratorians, with an emphasis on education to provide the best use of the test (Table 1). Despite the perception that the adoption of hs-cTn assays will universally increase the rate of myocardial injury and MI, it should be understood that not all cTn assays are equal and that the impact upon clinical implementation may be largely influenced by the assay and/or URL thresholds used both before and after implementation.
References
- 1. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581-98.
- 2. Wu AHB, Christenson RH, Greene DN, et al. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem2018;64:645-55.
- 3. Gunsolus IL, Jaffe AS, Sexter A, et al. Sex-specific 99th percentiles derived from the AACC Universal Sample Bank for the Roche Gen 5 cTnT assay: Comorbidities and statistical methods influence derivation of reference limits. Clin Biochem 2017;50:1073-7.
- 4. Apple FS, Sandoval Y, Jaffe AS. In Reply. Clin Chem 2017;63:1167-70.
- 5. Sandoval Y, Jaffe AS. Using High-Sensitivity Cardiac Troponin T for Acute Cardiac Care. Am J Med 2017;130:1358-65.
- 6. Twerenbold R, Boeddinghaus J, Nestelberger T, et al. Clinical Use of High-Sensitivity Cardiac Troponin in Patients With Suspected Myocardial Infarction. J Am Coll Cardiol 2017;70:996-1012.
- 7. Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J; IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin Chem 2017;63:73-81.
- 8. Apple FS, Jaffe AS, Collinson P, et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem 2015;48:201-3.
- 9. Sandoval Y, Smith SW, Apple FS. Present and Future of Cardiac Troponin in Clinical Practice: A Paradigm Shift to High-Sensitivity Assays. Am J Med 2016;129:354-65.
- 10. Reichlin T, Twerenbold R, Reiter M, et al. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med 2012;125:1205-13.
- 11. Twerenbold R, Jaeger C, Rubini Gimenez M, et al. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J 2016;37:3324-32.
- 12. Odqvist M, Andersson PO, Tygesen H, Eggers KM, Holzmann MJ. High-Sensitivity Troponins and Outcomes After Myocardial Infarction. J Am Coll Cardiol 2018;71:2616-24.
- 13. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 Myocardial Infarction and Myocardial Injury: Clinical Transition to High-Sensitivity Cardiac Troponin I. Am J Med 2017;130:1431-9.
- 14. Greenslade J, Cho E, Van Hise C, et al. Evaluating Rapid Rule-out of Acute Myocardial Infarction Using a High-Sensitivity Cardiac Troponin I Assay at Presentation. Clin Chem 2018;64:820-9.
- 15. Love SA, Sandoval Y, Smith SW, et al. Incidence of Undetectable, Measurable, and Increased Cardiac Troponin I Concentrations Above the 99th Percentile Using a High-Sensitivity vs a Contemporary Assay in Patients Presenting to the Emergency Department. Clin Chem 2016;62:1115-9.
- 16. Sandoval Y, Smith SW, Schulz KM, et al. Diagnosis of type 1 and type 2 myocardial infarction using a high-sensitivity cardiac troponin I assay with sex-specific 99th percentiles based on the third universal definition of myocardial infarction classification system. Clin Chem2015;61:657-63.
- 17. Grinstein J, Bonaca MP, Jarolim P, et al. Prognostic implications of low level cardiac troponin elevation using high-sensitivity cardiac troponin T. Clin Cardiol 2015;38:230-5.
- 18. Melki D, Lugnegård J, Alfredsson J, et al. Implications of Introducing High-Sensitivity Cardiac Troponin T Into Clinical Practice: Data From the SWEDEHEART Registry. J Am Coll Cardiol 2015;65:1655-64.
- 19. Sandoval Y, Thygesen K. Myocardial Infarction Type 2 and Myocardial Injury. Clin Chem 2017;63:101-7.
- 20. Nestelberger T, Boeddinghaus J, Badertscher P, et al. Effect of Definition on Incidence and Prognosis of Type 2 Myocardial Infarction. J Am Coll Cardiol 2017;70:1558-68.
- 21. Greenslade JH, Adikari T, Mueller C, et al. Characteristics and occurrence of type 2 myocardial infarction in emergency department patients: a prospective study. Emerg Med J 2017;35:168-75.
- 22. Sandoval Y, Thordsen SE, Smith SW, et al. Cardiac troponin changes to distinguish type 1 and type 2 myocardial infarction and 180-day mortality risk. Eur Heart J Acute Cardiovasc Care 2014;3:317-25.
- 23. Saenger AK, Haymond S. Utilization of cardiac troponin assays in adult and pediatric populations: Guideline recommendations vs. reality. Clin Biochem 2015;48:1213-8.
- 24. Bagai A, Alexander KP, Berger JS, et al. Use of troponin assay 99th percentile as the decision level for myocardial infarction diagnosis. Am Heart J 2017;190:135-9.
- 25. Collinson P, Hammerer-Lercher A, Suvisaari J, et al. How Well Do Laboratories Adhere to Recommended Clinical Guidelines for the Management of Myocardial Infarction: The CARdiac MArker Guidelines Uptake in Europe Study (CARMAGUE). Clin Chem2016;62:1264-71.
- 26. Hachey BJ, Kontos MC, Newby LK, et al. Trends in Use of Biomarker Protocols for the Evaluation of Possible Myocardial Infarction. J Am Heart Assoc 2017;6:e005852.
- 27. Shah AS, Griffiths M, Lee KK, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350:g7873.
- 28. Mills NL, Churchhouse AM, Lee KK, et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011;305;1210-6.
- 29. Shah AS, McAllister DA, Mills R, et al. Sensitive troponin assay and the classification of myocardial infarction. Am J Med 2015;128:493-501.
- 30. Shah ASV, Sandoval Y, Noaman A, et al. Patient selection for high sensitivity cardiac troponin testing and diagnosis of myocardial infarction: prospective cohort study. BMJ 2017;359:j4788.
- 31. Bjurman C, Larsson M, Johanson P, et al. Small changes in troponin T levels are common in patients with non-ST-segment elevation myocardial infarction and are linked to higher mortality. J Am Coll Cardiol 2013;62:1231-8.

http://www.cbsmd.cn Contact us by cbs@cbsmd.cn
Copyright ⓒ CBSMD Nanjing China. All rights reserved.



